(
In the last two years we have made an effort to unravel the photodesorption dynamics of ammonia molecules from various surfaces. Mike White and coworkers, Univ. of Texas at Austin, had in an attempt to nitride Gallium arsenide by photodissociating adsorbed ammonia (NH3) observed that the latter desorbed with high efficiency rather than to dissociate. Experiments comparing the efficiency for NH3 and its isotopomer, ND3, had indicated that vibrational excitation of the ammonia molecules upon photon excitation must mediate the efficient desorption. However, it had been accepted earlier that vibrational excitation of molecules is inefficient in desorption based on experiments by Chuang and coworkers at IBM Almaden research center, USA.
With this puzzle in mind we embarked on a study of the photodesorption of ammonia. We used a technique, called resonant multiphoton ionization (REMPI), to determine into which degrees of freedom, i.e. vibrations and rotations of the ammonia molecule, energy is channeled during the desorption process. We expected that this would reveal the mechanism of this desorption process.
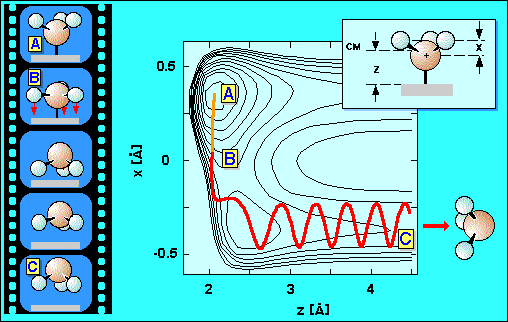
Ammonia in its electronic ground state has a pyramidal structure. Our experiments showed that most of the excess energy is channeled into the out-of-plane bending mode of the ammonia molecules. This bending mode is a motion in which the structure oscillates between being more planar and more pyramidal than the equilibrium geometry. Therefore it is commonly called "umbrella" mode.

(![]() You may also watch an animation of the desorption dynamics)
You may also watch an animation of the desorption dynamics)
Now it becomes clear how a vibration in the molecule is utilized to help the ammonia molecule to desorb and why this is exceptionally efficient. It is known from spectroscopy of the free molecule that, when electronically excited, it wants to adopt a planar structure. The same happens to the adsorbed ammonia molecule. Once electronically excited it starts to deform to become planar, i.e. the hydrogen atoms move to lie in one plane with the nitrogen atom. However, shortly after the hydrogen atoms start to move, the electronic excitation decays. In net effect the hydrogen atoms have become accelerated towards the surface. When the hydrogen atoms approach the surface strong repulsive forces arise between the electron cloud of the hydrogen atoms and the electrons located on the substrate atoms which pushes the whole molecule away from the surface. This scenario is illustrated in the animation above.
Experiments as described above reveal more that just the mechanism how electronic excitation leads to desorption. We can observe subtle effects which are reflected by how individual quantum states become populated. According to the rules of quantum mechanics each of the lower levels of the umbrella mode is split into two levels, of which one has a symmetric (blue in the figure below) and the other a antisymmetric (red) wavefunction. By the way, between these levels operates the astronomical ammonia maser.
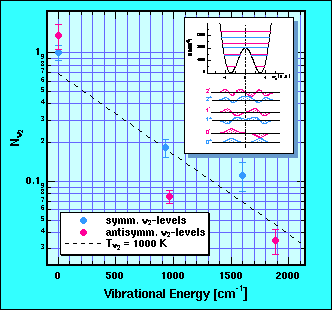
What we observe is a marked propensity for the symmetric levels to be stronger populated than the related antisymmetric ones. Only for the vibrational ground state are both equally populated. As one sees in the movie, in the classical picture the ammonia molecule leaves the surface with the hydrogen atoms pointing towards the substrate. In quantum language that implies that symmetric and anti-symmetric states have to be equally populated with a defined phase to each other. The experiment indicates that this is not quite true for the higher vibrational states. For these states some flux must also be present in the other geometry.
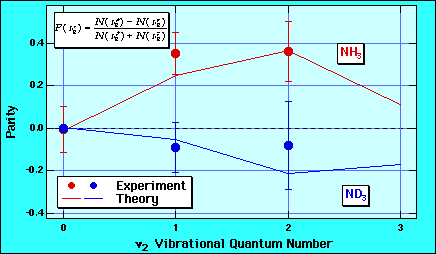
If one does the same experiment with ND3, one finds that the propensity for the symmetry states reverses. Now antisymmetric states are stronger populated. This is a reflection of the fact that in quantum mechanics the different states accumulate different phases while the particle desorbs. These phases depend on the velocity of the particle which is smaller for ND3. Interference between the different states determines the branching into the final eigenstates.
These experiments have also attracted the interest of theorists who performed model calculations. We have enjoyed collaboration with Peter Saalfrank, FU Berlin, Germany, and Stephen Holloway and George Darling, Univ. Liverpool, GB. We should not conclude without mentioning the related work by Alan Burns and coworkers at Sandia National Laboratories, USA, and H. Guo at Univ. of New Mexico, USA.
If you want to learn more, have a look at our recent publications.
 Eckart Hasselbrink
Eckart Hasselbrink